麦角硫因
功效研究
被证实的多种免疫调节作用:
改善睡眠质量
增强记忆
抗高血糖诱导的内皮损伤
抗皮肤光老化
一、改善睡眠质量
人体临床:每天摄入 20毫克 BIO-EGT,持续4周,可显著改善睡眠质量。
(Makoto Katsube .(2022);Food-derived antioxidant ergothioneine improves sleep difficulties in humans)
实验设计:随机、双盲、对照实验,共429名患有焦虑和睡眠问题的志愿者(40至75岁),经匹兹堡睡眠质量指数(PSQI)、雅典失眠量表(AIS)、状态-特质焦虑量表(STAI)问卷、睡眠效率(EEG)评分等剔除不符合入组条件的样本后共100人入组,随机分为麦角硫因组和安慰剂组,受试者每天服用20mg麦角硫因或安慰剂,持续4周。
结果:麦角硫因能显著改善睡眠困难,增加了非快速眼动睡眠,减少入睡潜伏期和入睡后醒来的频率。
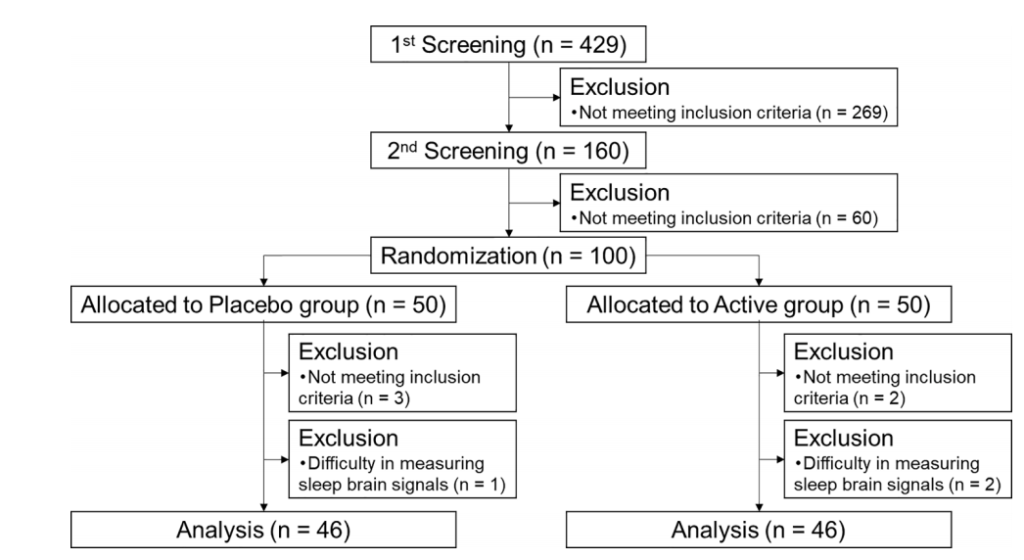
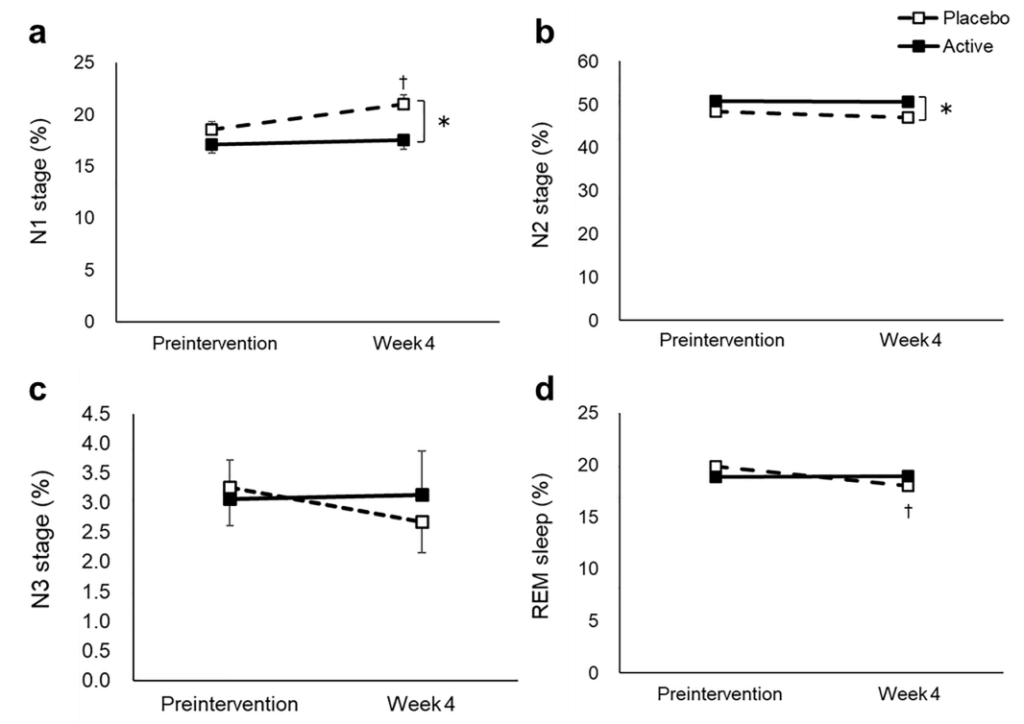
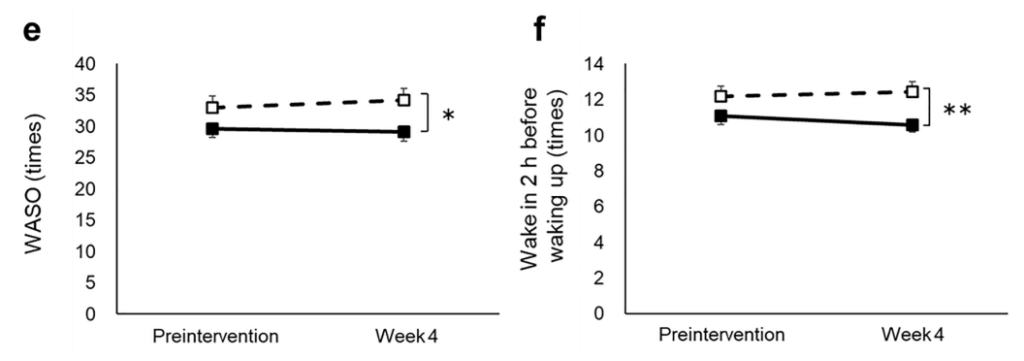
a N1 stage, b N2 stage, c N3 stage, d REM stage, e WASO, f wake in 2 h before waking up, g SWS latency, h sleep efficiency, i delta power during the first sleep cycle, and j total sleep time were measured before and after the intervention. The dotted and solid lines indicate the placebo (n = 46) and active group (n = 46), respectively. Mean values with standard error of the mean; **P < 0.01, *P < 0.05 (Welch t-test); † P < 0.05 versus baseline (paired t-test). EEG, electroencephalograph; SWS, slow wave sleep; REM, rapid eye movement; WASO, wake after sleep onset.
二、增强记忆
动物功效实验:ICR小鼠口服麦角硫因(剂量为1~50 mg/kg)后在新物体识别测试和空间识别测试的辨别指数均显著高于对照组。
(Noritaka Nakamichi.(2021);Oral Administration of the Food-Derived Hydrophilic Antioxidant Ergothioneine Enhances Object Recognition Memory in Mice)。
试验设计:ICR小鼠口服ERGO(剂量为1-50 mg/kg),每周三次,连续两周后;采用新目标识别试验、空间识别试验、LC-MS/MS、高尔基染色、神经元培养、western blotting、免疫细胞化学、RT-PCR等方法研究口服麦角硫因对正常小鼠物体识别记忆的增强作用。
结果:口服麦角硫因可增强对物体识别和记忆的能力,这种增强效果是通过促进海马神经元成熟来实现的。
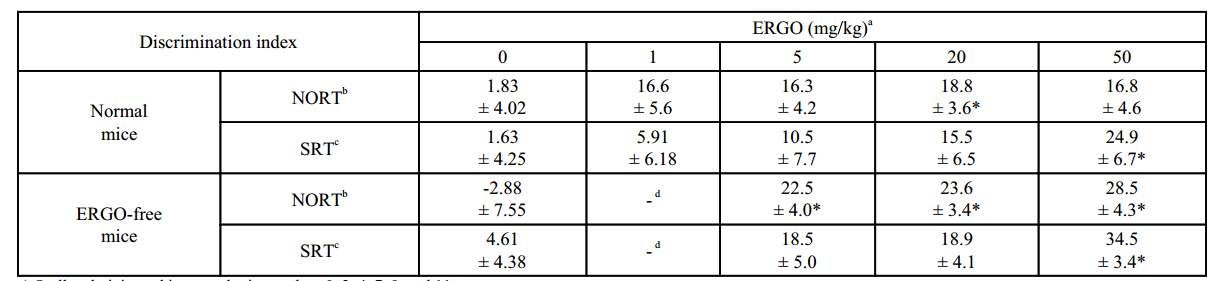
a) Orally administered in normal mice on days 0, 2, 4, 7, 9, and 11
b) Performed at three days after the last ERGO administration
c) Performed at six days after the last ERGO administration
d) Not performed
* Significant difference from the corresponding control values (P < 0.05)
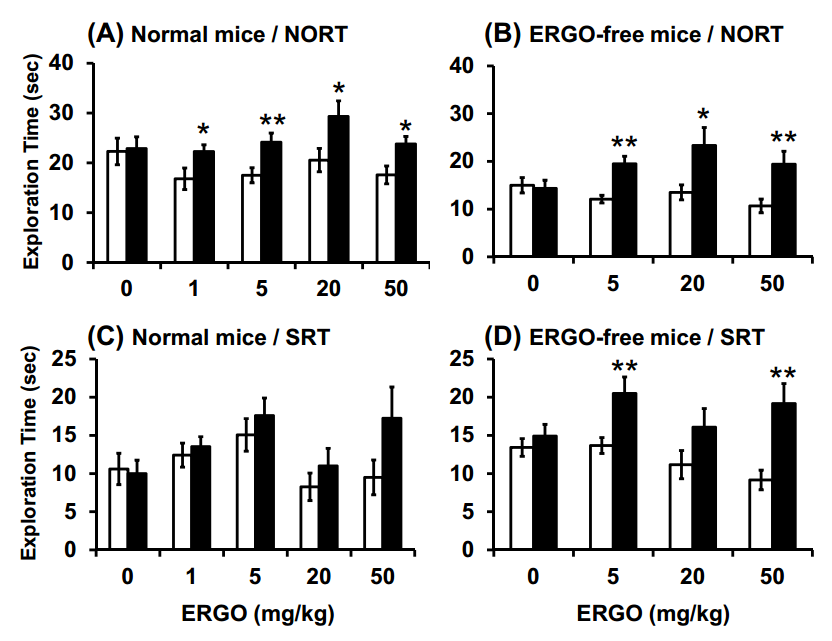
Fig. (1). Effect of oral administration of ERGO on object recognition and object location memory under normal conditions.
Normal (A, C) and ERGO-free (B, D) mice were orally administered ERGO at 0, 1, 5, 20, or 50 mg/kg on days 0, 2, 4, 7, 9, and 11. Three and six days after the final ERGO administration, NORT (A, B) and SRT (C, D) were conducted, respectively, and the exploration time was measured. The white and black columns in panels (A) and (B) display the exploration time for the familiar and novel objects, respectively.
The white and black columns in panels (C) and (D) show the exploration time for the unmoved and moved objects, respectively. Each value represents the mean ± S.E.M. (n = 12–15). * Significant difference from the control (P < 0.05); ** Significant difference from the control (P < 0.01).
三、有效保护高血糖诱导的内皮细胞衰老
细胞实验:麦角硫因能够通过调节SIRT1和SIRT6信号传导干扰与高血糖相关的内皮细胞衰老,对2型糖尿病中高血糖诱发的氧化损伤起到保护作用,降低心血管疾病的风险。
(Nunzia D’Onofrio.(2016); Ergothioneine oxidation in the protection against high-glucose induced endothelial senescence: Involvement of SIRT1 and SIRT6)
实验设计:血管内皮细胞在梯度浓度的麦角硫因溶液(0.01-1.00 mM)中孵育12小时,然后在高葡萄糖(25 mM)中孵育48小时。细胞评价表明,0.5 mM浓度的麦角硫因就可有效防止高糖诱发细胞毒性的作用。
结论:麦角硫因能够抵御高血糖诱发的血管内皮细胞衰老,降低心血管疾病风险。
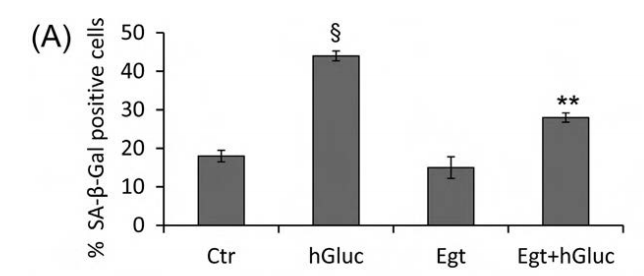
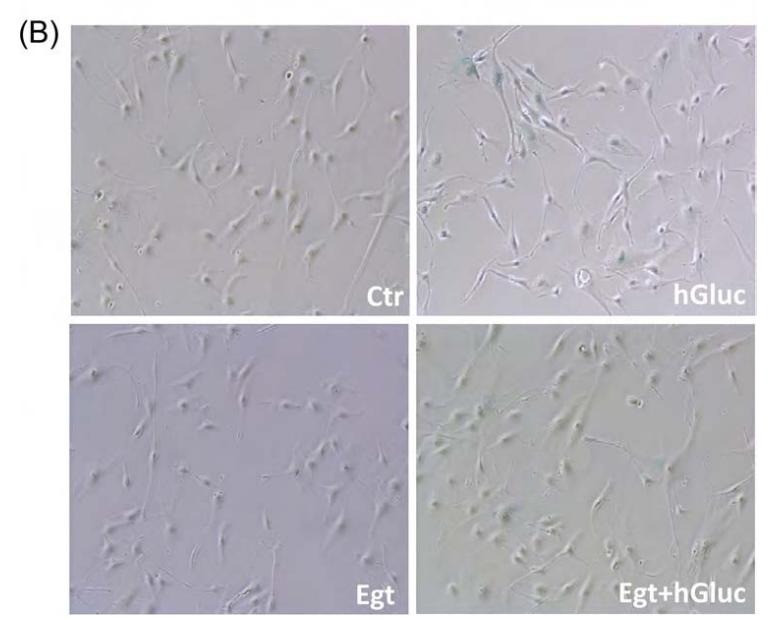
Fig. 3. Effect of ergothioneine on high-glucose induced EC senescence. (A) Percentage of SA-β-galactosidase staining positive EC grown for 48 h with high-glucose (hGluc), high-glucose plus ergothioneine (0.5 mM) (EgtþhGluc), ergothioneine (Egt) (0.5 mM), or media containing nGluc (Ctr). (B) Representative images of SA-β-galactosidase staining (blue). Data are the mean7SD of five independent experiments with **Po0.01 vs. hGluc and § Po0.01 vs. Ctr.
四、麦角硫因抗皮肤光老化
细胞实验:麦角硫因通过激活角质形成细胞的Nrf2/HO-1通路和HSP70,减轻UVB损伤引起的皮肤成纤维细胞衰老。
(Hyun Ju Ko.(2021);Ergothioneine alleviates senescence of fibroblasts induced by UVB damage of keratinocytes via activation of the Nrf2/HO-1 pathway and HSP70 in keratinocytes)
实验设计:角质形成细胞用麦角硫因预处理后用UVB照射,评估ROS的产生和凋亡,还通过Western blotting和real-time PCR分析了Nrf2/HO-1通路、HSP70、促凋亡蛋白和旁分泌细胞因子。胶原降解相关基因和衰老也进行了评估。
结果:麦角硫因预处理角质形成细胞显著抑制Nrf2/HO-1通路和HSP70的下调,并通过抑制ROS的产生和促凋亡蛋白(包括caspase-8和PARP)的裂解来保护角质形成细胞。此外,麦角硫因显著降低旁分泌细胞因子,包括IL-1β、IL-6和TNF-α。在与麦角硫因处理的角质形成细胞共培养的成纤维细胞中,胶原降解相关基因的表达水平和成纤维细胞衰老显著降低;而I型前胶原的合成则显著增加。
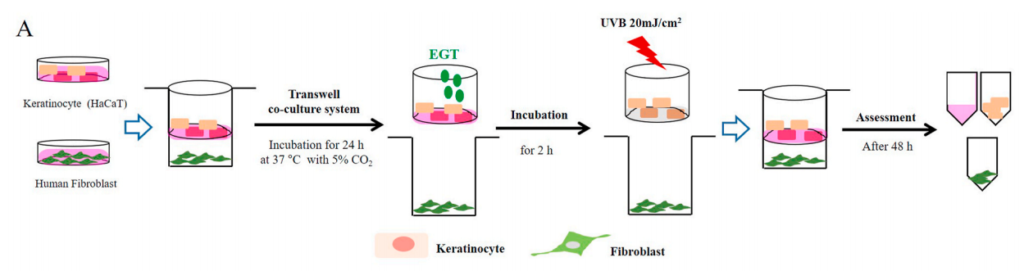
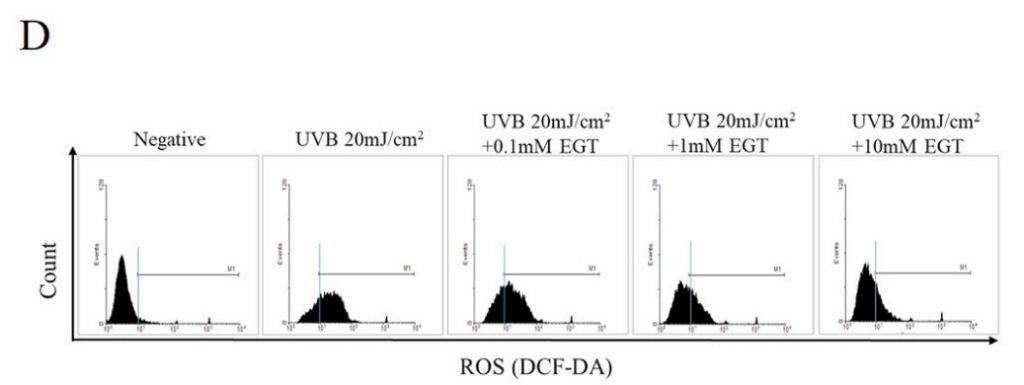
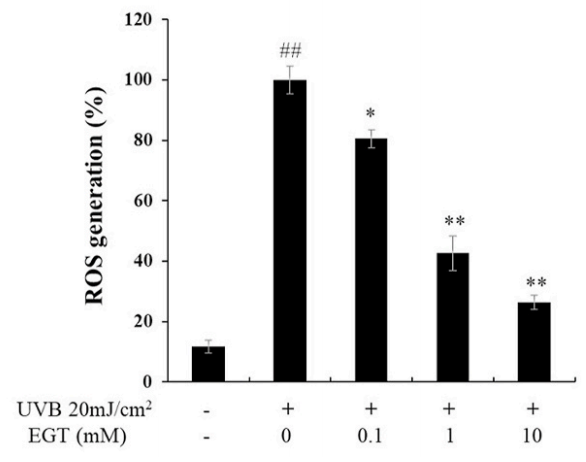
Fig. 1. Effects of ergothioneine (EGT) on keratinocyte viability and oxidative stress in keratinocytes following ultraviolet B (UVB) exposure using a co-culture system containing keratinocytes and fibroblasts.
(A) Keratinocytes in the upper chamber and fibroblasts in the lower chamber were incubated for 24 h at 37 ◦C under 5% (v/ v) CO2. Keratinocytes with or without EGT pretreatment for 2 h were stimulated with UVB (20 mJ/cm2 ). Stimulated keratinocytes with or without EGT were cocultured with fibroblasts. After 48 h, the culture media and cells were harvested.
D) UVB-induced intracellular reactive oxygen species (ROS) levels in keratinocytes.
EGT inhibited ROS synthesis in keratinocytes following UVB irradiation. #p < 0.05 vs. negative control; *p < 0.05, **p < 0.01 vs. vehicle-treated keratinocytes.
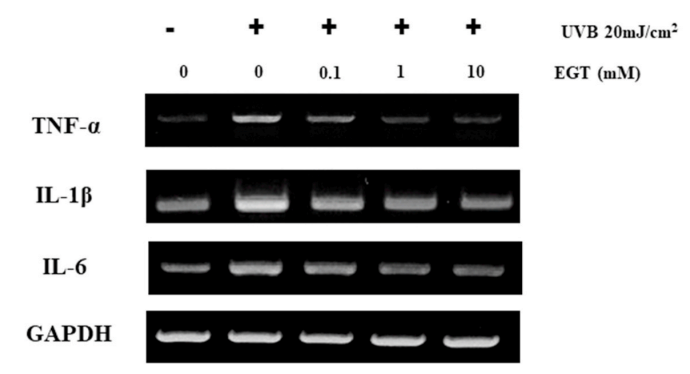
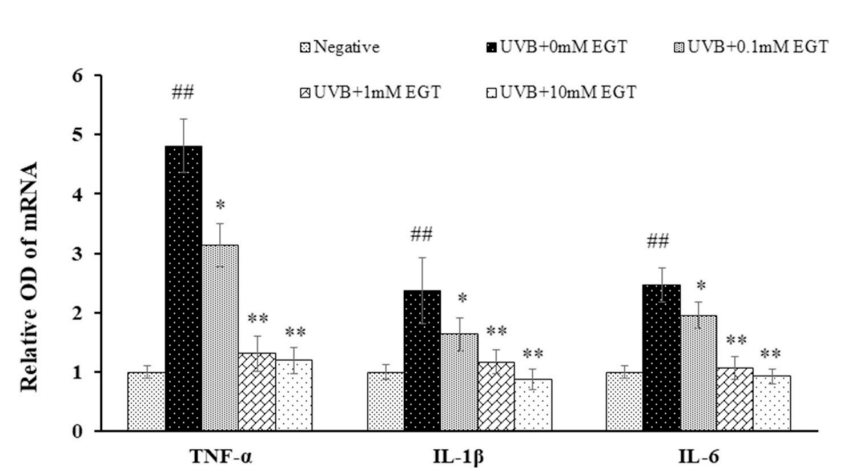
Fig. 5. EGT-mediated suppression of UVB-induced production of pro-inflammatory cytokines in keratinocytes. The mRNA levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in UVB-irradiated keratinocytes with and without EGT treatment were examined by RT-PCR. GAPDH was used as an internal control. Quantification of pro-inflammatory cytokine expression following normalization to GAPDH. #p < 0.05 vs. negative control; *p < 0.05, **p < 0.01 vs.vehicle-treated keratinocytes.
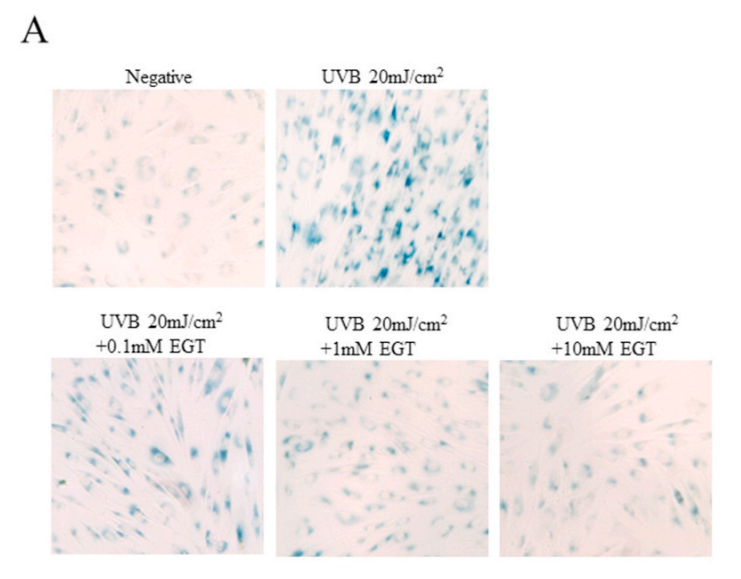
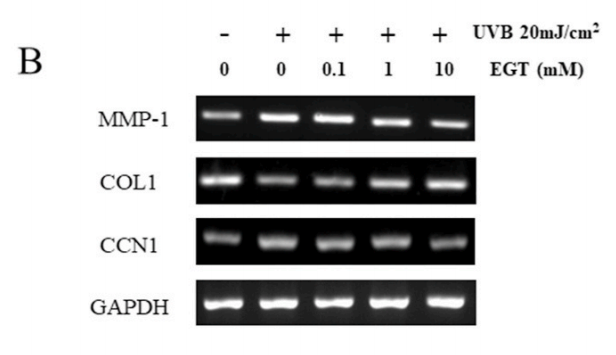
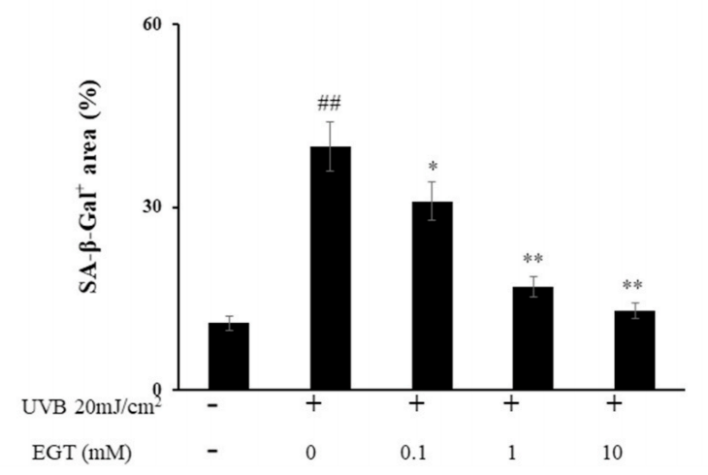
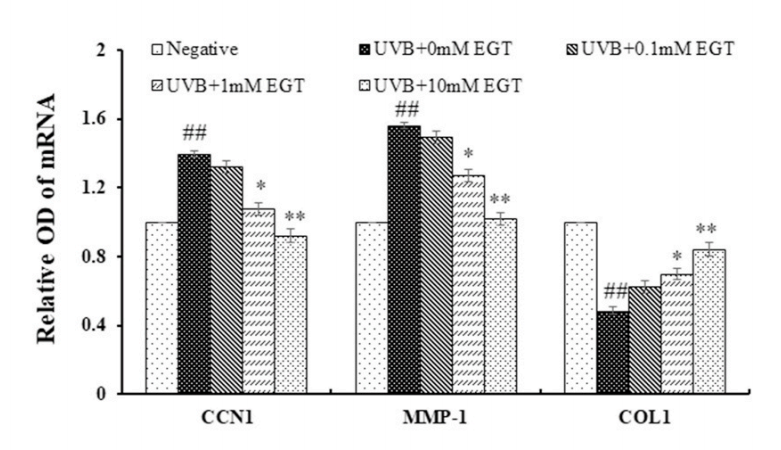
Fig. 6. Effect of EGT pretreatment of keratinocytes on UVB-induced photoaging in fibroblasts. (A) Fibroblasts were assessed by SA-β-gal staining. Quantitative analyses of senescent cells are indicated by the percentage of positive staining compared to the vehicle-treated group. (B) mRNA levels of collagen homeostasisassociated proteins (MMP-1, COL1, and CCN1) were quantified by RT-PCR and normalized to GAPDH as an internal control. ##p < 0.01 vs. negative control; *p < 0.05, **p < 0.01 vs. fibroblasts with vehicle-treated keratinocytes.
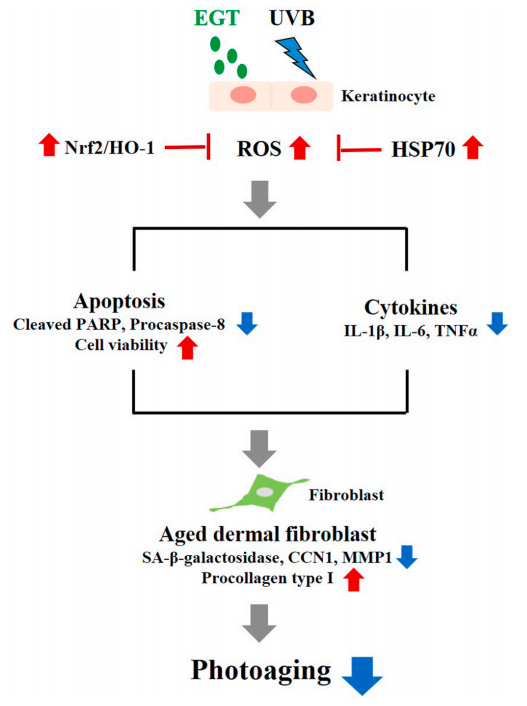
Fig. 7. Schematic illustration of the anti-photoaging effects of EGT in a keratinocyte/fibroblast co-culture system following UVB irradiation. UVB-irradiated keratinocytes promoted ROS synthesis, apoptosis, and production of proinflammatory cytokines. Furthermore, fibroblasts co-cultured with UVBirradiated keratinocytes showed changes in MMP-1, CCN1, and procollagen type I. The anti-photoaging effects of EGT in UVB-irradiated skin damage was mediated by suppression of the upregulation of ROS, pro-inflammatory cytokines, and keratinocyte apoptosis, and by maintaining collagen homeostasis in fibroblasts. CCN1, cysteine-rich protein 61; EGT, ergothioneine; HSP, heat shock protein; IL, interleukin; MMP, matrix metalloproteinase; ROS, reactive oxygen species; TNF, tumor necrosis factor; UVB, ultraviolet B.